Heyy guys! :)
Today, I gave a Ted-style talk to my anatomy class about my project. 20 Time was a fantastic experience, and I really hope it becomes the future of education. I'm very thankful to Mr.Orre and this class for implementing this idea.
As for my saltwater flashlight, I have reached an exciting conclusion with the circuit-building stage of my project! After adding more cups of saltwater in series, the light finally..lighted! :) Here is a video of it: https://youtu.be/YZbzsG0Vo7M\
I have not yet finished making my flashlight, but I have drawn a diagram of what my prototype will most likely look like. [Shown below]. It is basically just 4(or more) LEDs at the front, connected in series to four(or more) compartments of saltwater.
Though I haven't finished, I am still happy about where I've reached in this project. Making the circuit was probably the most important part of the project, and I am done with it! Now all I have to do is figure out a way to make the whole set-up MUCH smaller so it can fit in a portable flashlight. The main thing I have learnt through this project is patience. Even after many tries, my LED did not light up, and it took a lot of patience and willpower to keep going and not stop. And it all payed off because the LED lighted in the end!
Through my journey with this 20 Time project, I also realized that there were many ways that I could connect my physics-based project back to anatomy! It turns out that blood is a very ionic substance, as shown by the chart below. And of course, when ions flow, like blood does all the time, electricity can be created! Some potential uses for creating electricity from our blood I brainstormed were:
(1) To charge the human body when it is weak or tired
(2) To keep the heart in rhythm, instead of using a pacemaker(which patients have to constantly replace)
(3) Charge bones for patients of osteoporosis, etc.
(4) Many more.... :)
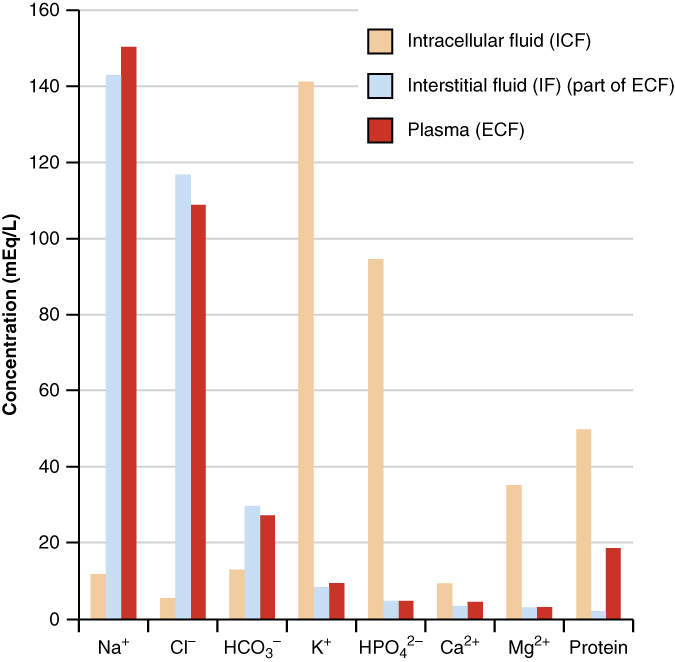
I think this really shows how interwoven all the sciences are these days, and the best of discoveries can be made when these beautiful sciences are combined.
The only thing I would have liked changed/added to this 20 Time project is more time. We had all set such ambitious goals, but most of us did not get to finish our projects because it was tough to find time amidst all the other schoolwork, sadly...
The Ted Talk I gave went really well! It was a good experience sharing everything I had done with all my classmates and my teacher. I definitely learnt how to make an effective powerpoint presentation with more showing (pictures) than telling.
Today, I gave a Ted-style talk to my anatomy class about my project. 20 Time was a fantastic experience, and I really hope it becomes the future of education. I'm very thankful to Mr.Orre and this class for implementing this idea.
As for my saltwater flashlight, I have reached an exciting conclusion with the circuit-building stage of my project! After adding more cups of saltwater in series, the light finally..lighted! :) Here is a video of it: https://youtu.be/YZbzsG0Vo7M\
I have not yet finished making my flashlight, but I have drawn a diagram of what my prototype will most likely look like. [Shown below]. It is basically just 4(or more) LEDs at the front, connected in series to four(or more) compartments of saltwater.
Though I haven't finished, I am still happy about where I've reached in this project. Making the circuit was probably the most important part of the project, and I am done with it! Now all I have to do is figure out a way to make the whole set-up MUCH smaller so it can fit in a portable flashlight. The main thing I have learnt through this project is patience. Even after many tries, my LED did not light up, and it took a lot of patience and willpower to keep going and not stop. And it all payed off because the LED lighted in the end!
Through my journey with this 20 Time project, I also realized that there were many ways that I could connect my physics-based project back to anatomy! It turns out that blood is a very ionic substance, as shown by the chart below. And of course, when ions flow, like blood does all the time, electricity can be created! Some potential uses for creating electricity from our blood I brainstormed were:
(1) To charge the human body when it is weak or tired
(2) To keep the heart in rhythm, instead of using a pacemaker(which patients have to constantly replace)
(3) Charge bones for patients of osteoporosis, etc.
(4) Many more.... :)
I think this really shows how interwoven all the sciences are these days, and the best of discoveries can be made when these beautiful sciences are combined.
The only thing I would have liked changed/added to this 20 Time project is more time. We had all set such ambitious goals, but most of us did not get to finish our projects because it was tough to find time amidst all the other schoolwork, sadly...
The Ted Talk I gave went really well! It was a good experience sharing everything I had done with all my classmates and my teacher. I definitely learnt how to make an effective powerpoint presentation with more showing (pictures) than telling.



